Introduction to therapeutic drug concentration monitoring
Therapeutic drug monitoring (TDM) is a pharmaceutical technical method that uses modern analytical technology to determine the concentration of drugs and their metabolites in patients' body fluids (mainly blood), so as to optimize the design or adjust the administration scheme, realize individualized treatment, improve curative effect and reduce toxic and side effects.
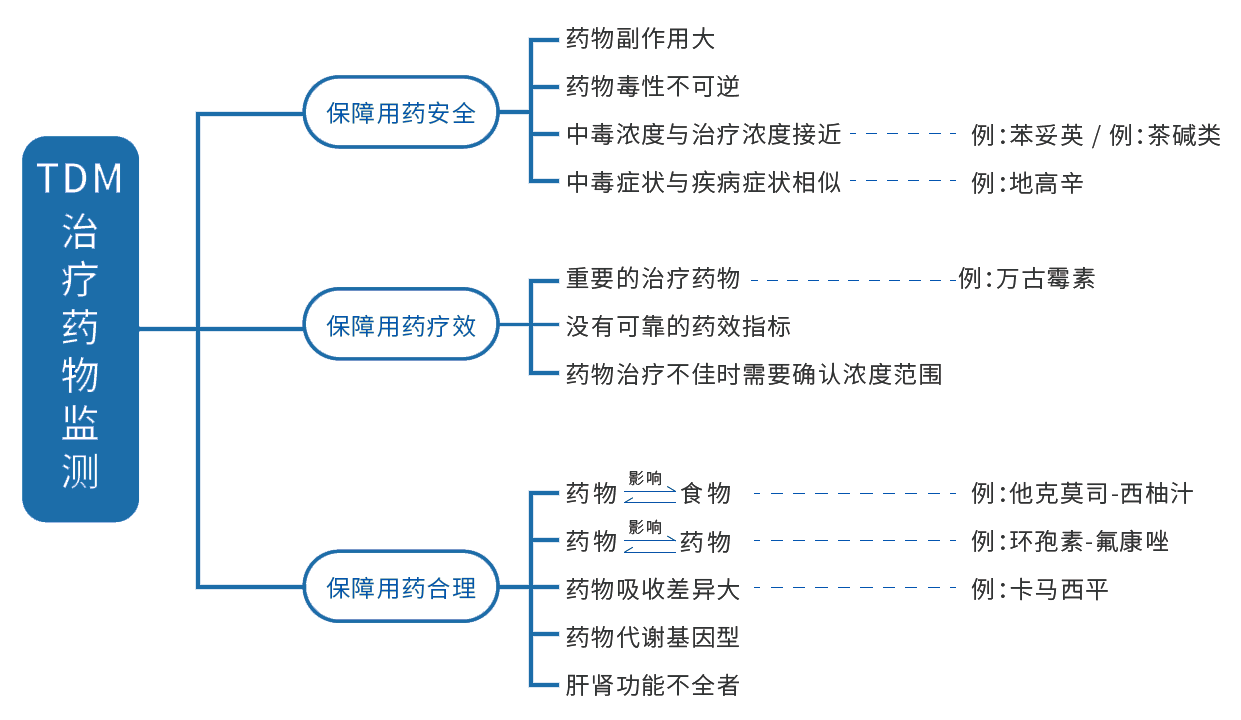
Clinical significance
Characteristics and testing index of the kit
High throughput: 1 needle of multiple drugs detected, 6 minutes / sample
High stability: standard curve can be established for one month
Strong specificity: differentiation and quantification of technical drugs and their metabolites
Strong compatibility: applicable instrument brands: SCIEX, Agilent, waters, Shimadzu
Suitable population
(1) Drugs that must undergo TDM for safety reasons (eg. carbamazepine)
(2) Suspected complete or partial non-compliance with medication
(3) Dose optimization after starting medication or changing dose
(4) Poor clinical efficacy at the recommended dose
(5) When the treatment was effective at the recommended dose, there were adverse reactions
(6) Concomitant medication has or is suspected of drug interaction
(7) TDM in pharmacovigilance project
(8) Prevention of recurrence under maintenance therapy
(9) Disease recurrence in case of adequate dosage
(10) There are gene mutations related to drug metabolism (gene deletion, gene polyploidy)
(11) Pregnant or lactating female patients
(12) Children and adolescent patients
(13) Elderly patients (> 65 years old)
(14) Patients with intellectual disabilities
(15) With pharmacokinetic related complications (liver or kidney dysfunction, cardiovascular disease)
(16) Law related patients
(17) Problems occurred after the original drug was changed to generic drug or generic drug was changed to original drug

